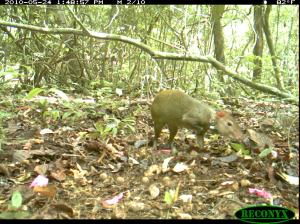
We all know the feeling – sometimes there just aren’t enough hours in the day to get all our work done. Such is the life of agoutis living in low-quality territories, who have to scrounge around the rainforest floor not only for today’s meal, but also to find seeds they can cache underground for the late rainy season when there will be even less food. To push ourselves as deadlines approach we set the alarm extra early, and have an extra cup of coffee to keep working later. Our latest discovery shows that hungry agoutis also stretch the hours of their day, but face much more dire consequences than a short-night’s sleep.
Our new paper published today in Animal Behavior, led by Lennart Suselbeek from Wageningen University, shows that hungry agoutis that wake up early or stay up late are much more likely to be eaten by ocelots, while those who have plenty of food sleep in and avoid the dangerous dusk and dawn periods.
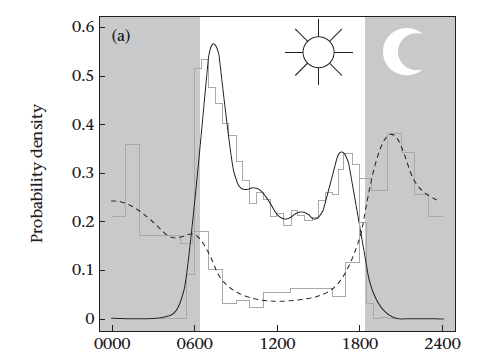
We used radio-collars and camera traps to measure animal activity. The camera data showed the expected overall patterns of the population in general, with agoutis out in the day (95% of photos) and ocelots working the night-shift (78% of photos).
However, there was some overlap, especially at dusk and dawn. Comparing the ratio of ocelot:agouti pictures at different times of day puts a number on this risk. During the day we’d get 500 agouti photos for every one ocelot picture – this is a safe time for agoutis to roam around with a low chance of running into a predator. At night this flips to 30 ocelots for every 1 agouti – this is the dangerous time when ocelots are on the prowl and agoutis are hiding in their dens (as we showed in another recent paper).
We also radio-tracked agoutis and used our Automated Radio Telemetry System to monitor them 24/7. The live data stream alerted us when animals died, giving a flat-line on the activity graph, and giving us a chance to run out and try to determine the cause of death. Most of these (17/19) were confirmed as ocelot kills. What more, we were able to pinpoint the time of day that the kill occurred, confirming that the dark hours of the night, especially dusk and dawn, are deadly for agoutis.
If the in-between periods are so important, why would an agouti get up before the sun was up, or stay up into the evening, when they must know ocelots are still active? This required us to focus in on individual agoutis, not general population-wide surveys. First, we looked at our radio-collared animals to see when they started and ended activity based on the radio signal. Then, to get more specific, we also put a camera trap on their burrows, to see exactly when they went in and out of their bedroom. They key part of this comparison was that we knew how much food each animal had available in their home range from our tree surveys. The results were simple but striking.
Both methods showed that agoutis in areas with less food left their burrows earlier and entered their burrows later than agoutis in food-rich areas. Hungry agoutis were much more active at twilight, and were more likely to get killed by an ocelot.
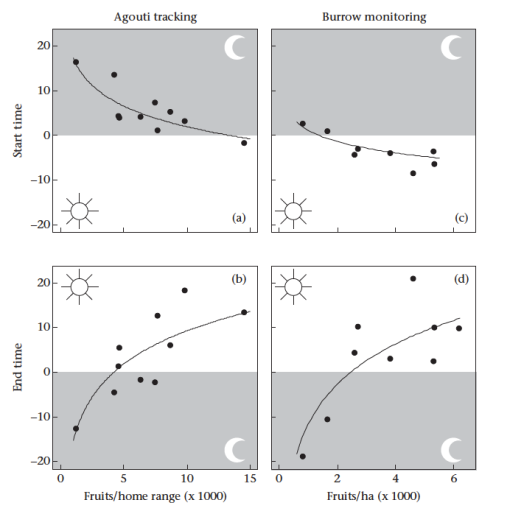
Start and end of activity by agoutis related to the food they had available to them, showing that hungry agoutis are more likely to be active when its dark.
In the end it’s the predator that delivers the final blow, but this shows that hunger is driving them to take risks, and highlights the importance of having a good quality territory with lots of food.
So agoutis are kind of like us, busy mammals with too much to do, and not enough time in the day. The next time your alarm seems too early, and the sky is still dark outside, consider the chances there is an ocelot outside your bedroom – maybe its ok to hit snooze one more time.